STUDIES TRIALS APPRAISALS _______________________________________
Nephrology, Cardiology & Internal Medicine for students, doctors and faculty
______________________________________
STUDIES, TRIALS and Critical Appraisals
Section A: Study and Trial
- ADPKD
- AKI
- Non-AKI ICU cases
- Cardiorenal AKI
- ANCA Vasculitis
- Anaemia
- Hyperkalaemia
- Lupus nephritis
- GN, IGAN
- CKD DN
- Hypertension
- Haemodialysis
- Peritoneal dialysis
- Transplantation
- Gout and MBD
Section B: Critical appraisal of RCT
- Lupus nephritis (Glassok)
- ADPKD (Mark)
- ANCA Vasculitis (Khawja)
- AKI (Levin)
- Primary GN (Glassok)
- CKD (Nahas)
- Anaemia (Kossi)
- Lipid (Kossi)
- DN (Meguud)
- MBD (Mohsen)
- Haemodialysis (Charles)
- Peritoneal dialysis (Davies)
- Transplant (Mattar)
_____________________________________
______________________________________
Section A: Studies and trials
A1-ADPKD
HALT study, 2012 KI. Analysis of parameters. In summary, this analysis 1) confirms a strong association between renal volume and functional parameters, 2) shows that gender and other factors differentially affect the development of polycystic disease in the kidney and liver, and 3) suggests an association between anthropomorphic measures reflecting preand/or post-natal growth and the severity of the disease.
ALADIN TRIAL, 2013, Somatostatin in PKD. In summary, difference was not significant (p=0·25). 37 (92·5%) participants in the octreotide-LAR group and 32 (82·1%) in the placebo group had at least one adverse event (p=0·16). Participants with serious adverse events were similarly distributed in the two treatment groups.
RAPYD STUDY, 2012, Rapamycin in PKD type 1. In summary, rapamycin does not influence the progression of type I ADPKD, although the higher drug dose tested prevented both the increase in kidney volume and the worsening of renal function.
Low dose Sirolimus in PKD 2014In summary, Patients with ADPKD receiving LD rapamycin demonstrated a significant increase in iGFR compared with those receiving standard care, without a significant effect on TKV after 12 months.
ERA-EDTA on ADPKD 2019. Summary: Although first reported 500 years ago, this disorder is still regarded as untreatable and its pathogenesis is poorly understood despite much study. During the past 40 years, however, remarkable advances have transformed our understanding of how the disease develops and have led to rapid changes in diagnosis, prognosis, and treatment, especially during the past decade. This Review will summarise the key findings, highlight recent developments, and look ahead to the changes in clinical practice that will likely arise from the adoption of a new management framework for this major kidney disease.
Study on ADPKD Criteria. Unified criteria: Individuals who are at risk for autosomal dominant polycystic kidney disease are often screened by ultra- sound.
DYPEK trial. Somatostatin (SST), a hormone that is involved in many cell processes, has the ability to inhibit intracellular cAMP production. However, SST itself has limited therapeutic potential since it is rapidly eliminated in vivo. Therefore analogues have been synthesized, which have a longer half-life and may be promising agents in the treatment of ADPKD. This review provides an overview of the complex physiological effects of SST, in particular renal, and the potential therapeutic role of SST analogues in ADPKD.
_______________________________________
A2-AKI
KDIGO summary on Contrast induced AKI
PICARD trial--CRRT & AKI survival. Among critically ill patients with AKI, initiation of dialysis at higher BUN concentrations was associated with an increased risk for death.
IDEAL-ICU trial 2018. Early vs late initiation of CRRT :: patients with septic shock who had severe acute kidney injury, there was no significant difference in overall mortality at 90 days between patients who were assigned to an early strategy for the initiation of renal-replacement therapy and those who were assigned to a delayed strategy.
STARRT AKI trial. In patients with severe acute kidney injury (AKI) but no urgent indication for renal replacement therapy (RRT), the optimal time to initiate RRT remains controversial. While starting RRT preemptively may have benefits, this may expose patients to unnecessary RRT. To study this, we conducted a 12-center open-label pilot trial of critically ill adults with volume replete severe AKI. Patients were randomized to accelerated (12h or less from eligibility) or standard RRT initiation. No safety signal was evident in either arm. Our findings can inform the design of a large-scale effectiveness randomized control trial.
AKI prospective outcome study--with urinary proteomic analysis. We emphasize that in clinical AKI, the pattern of proteinuria, as analyzed by SDS-PAGE, might indicate diagnostic significance in terms of glomerular or tubular origin of AKI, and the pattern of proteinuria, particularly glomerular predominance, could predict progression to CRF/ESRF in the long run.
IMPROVE AKI TRIAL--ongoing 2018. Use of radio-contrast dye in coronary intervention, there is a possibility of damage to that person's kidneys, which could result in being on dialysis or early death. The investigators are testing novel coaching and automated tools to help healthcare teams apply approaches that have been shown to prevent damage to kidneys during a cardiac catheterization procedure.
REVERSE AKI (copleted) Brief summary: Observational studies among patients with acute kidney injury (AKI) have shown an association with fluid accumulation and increased mortality.
The objective if this study is to determine whether a fluid restrictive treatment regimen will lead to a lower cumulative fluid balance at 72 hours from randomization in critically ill patients with AKI and whether this approach is safe and feasible.
Result: In critically ill patients with AKI, a restrictive fluid management regimen resulted in lower cumulative fluid balance and less adverse events compared to usual care. Larger trials of this intervention are justified.
RENAL TRIAL AKI. Intensity of Continuous Renal-Replacement Therapy in Critically Ill PatientsList of authors.The RENAL Replacement Therapy. CONCLUSIONS: In critically ill patients with acute kidney injury, treatment with higher-intensity continuous renal-replacement therapy did not reduce mortality at 90 days.
ELAINE AKI TRIAL France, 2016. Among critically ill patients with AKI, early RRT compared with delayed initiation of RRT reduced mortality over the first 90 days. Further multicenter trials of this intervention are warranted.
AKIKI TRIAL AKI, 2016. No significant difference with regard to mortality between an early and a delayed strategy for the initiation of renal-replacement therapy. A delayed strategy averted the need for renal-replacement therapy in an appreciable number of patients.
SALT-ED, Low Chloride Balance solution instead of NS in non-critical patients. NS vs Low Chloride solution in NON-ICU : in non-critical patients: No difference in mortality and outcome.
SMART Trial. Low Chloride solution vs NS in critically ill patient. Conclusions: Among critically ill adults, the use of balanced crystalloids for intravenous fluid administration resulted in a lower rate of the composite outcome of death from any cause, new renal-replacement therapy, or persistent renal dysfunction than the use of saline.
_____________________________________
A3. NON-AKI
BALANCE, Normal Saline vs low Chloride solution in ICU :: No difference in mortality or recovery.
SALT-ED NS vs Low Chloride solution in NON-ICU. :: No difference in mortality and outcome.
_____________________________________
Table from AJKD core curriculum on a few important studies on AKI
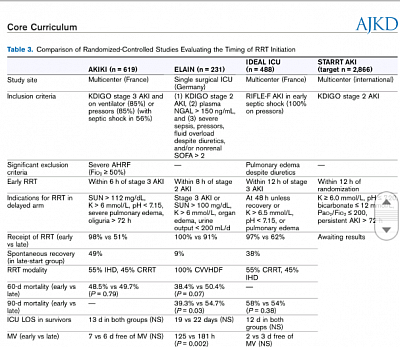
ACS, abdominal compartment syndrome; AKI, acute kidney injury; ALF, acute liver failure; CRS, cardiorenal syndrome; CSA-AKI, cardiac surgery–associated acute kidney injury; HF, heart failure; HRS, hepatorenal syndrome; HTN, hypertension; IAH, intra-abdominal hypertension; IAP, intra-arterial pressure; ICU, intensive care unit; INR, international normalized ratio; KDIGO, Kidney Disease: Improving Global Outcomes; MAP, mean arterial pressure; qSOFA, quick Sequential Organ Failure Assessment; Scr, serum creatinine; SOFA, Sequential Organ Failure Assessment; TIPS, transjugular intrahepatic portosytemic shunt; TMA, thrombotic microangiopathy.
_______________________________________
A4. CARDIORENAL SYNDROME
Low-Dose Dopamine or Low-Dose Nesiritide in Acute Heart Failure With Renal DysfunctionThe ROSE Acute Heart Failure Randomized Trial
Importance Small studies suggest that low-dose dopamine or low-dose nesiritide may enhance decongestion and preserve renal function in patients with acute heart failure and renal dysfunction; however, neither strategy has been rigorously tested. Objective To test the 2 independent hypotheses that, compared with placebo, addition of low-dose dopamine (2 μg/kg/min) or low-dose nesiritide (0.005 μg/kg/min without bolus) to diuretic therapy will enhance decongestion and preserve renal function in patients with acute heart failure and renal dysfunction. Design, Setting, and Participants Multicenter, double-blind, placebo-controlled clinical trial (Renal Optimization Strategies Evaluation [ROSE]) of 360 hospitalized patients with acute heart failure and renal dysfunction (estimated glomerular filtration rate of 15-60 mL/min/1.73 m2), randomized within 24 hours of admission. Enrollment occurred from September 2010 to March 2013 across 26 sites in North America. Interventions Participants were randomized in an open, 1:1 allocation ratio to the dopamine or nesiritide strategy. Within each strategy, participants were randomized in a double-blind, 2:1 ratio to active treatment or placebo. The dopamine (n = 122) and nesiritide (n = 119) groups were independently compared with the pooled placebo group (n = 119). Main Outcomes and Measures Coprimary end points included 72-hour cumulative urine volume (decongestion end point) and the change in serum cystatin C from enrollment to 72 hours (renal function end point). Results Compared with placebo, low-dose dopamine had no significant effect on 72-hour cumulative urine volume (dopamine, 8524 mL; 95% CI, 7917-9131 vs placebo, 8296 mL; 95% CI, 7762-8830 ; difference, 229 mL; 95% CI, −714 to 1171 mL; P = .59) or on the change in cystatin C level (dopamine, 0.12 mg/L; 95% CI, 0.06-0.18 vs placebo, 0.11 mg/L; 95% CI, 0.06-0.16; difference, 0.01; 95% CI, −0.08 to 0.10; P = .72). Similarly, low-dose nesiritide had no significant effect on 72-hour cumulative urine volume (nesiritide, 8574 mL; 95% CI, 8014-9134 vs placebo, 8296mL; 95% CI, 7762-8830; difference, 279 mL; 95% CI, −618 to 1176 mL; P = .49) or on the change in cystatin C level (nesiritide, 0.07 mg/L; 95% CI, 0.01-0.13 vs placebo, 0.11 mg/L; 95% CI, 0.06-0.16; difference, −0.04; 95% CI, −0.13 to 0.05; P = .36). Compared with placebo, there was no effect of low-dose dopamine or nesiritide on secondary end points reflective of decongestion, renal function, or clinical outcomes. Conclusion and Relevance In participants with acute heart failure and renal dysfunction, neither low-dose dopamine nor low-dose nesiritide enhanced decongestion or improved renal function when added to diuretic therapy.
Cardiorenal rescue study in acute decompensated heart failure: rationale and design of CARRESS-HF, for the Heart Failure Clinical Research NetworkBradley A Bart et al. J Card Fail. 2012 Mar.
Background: Worsening renal function is common among patients hospitalized for acute decompensated heart failure (ADHF). When this occurs, subsequent management decisions often pit the desire for effective decongestion against concerns about further worsening renal function. There are no evidence-based treatments or guidelines to assist in these difficult management decisions. Ultrafiltration is a potentially attractive alternative to loop diuretics for the management of fluid overload in patients with ADHF and worsening renal function.
Methods and results: The National Heart, Lung, and Blood Institute Heart Failure Clinical Research Network designed a clinical trial to determine if ultrafiltration results in improved renal function and relief of congestion compared with stepped pharmacologic care when assessed 96 hours after randomization in patients with ADHF and cardiorenal syndrome. Enrollment began in June 2008. This paper describes the rationale and design of the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF).
Conclusions: Treating the signs and symptoms of congestion in ADHF is often complicated by worsening renal function. CARRESS-HF compares treatment strategies (ultrafiltration vs stepped pharmacologic care) for the management of worsening renal function in patients with ADHF. The results of the CARRESS-HF trial are expected to provide information and evidence as to the most appropriate approaches for treating this challenging patient population.
________________________________
A5. ANCA associated vasculitIS
ADVOCATE trial Avacopan Shows Superiority Over Steroids to Treat AAV in Phase 3 ADVOCATE Trial.
The pivotal ADVOCATE trial (NCT02994927) met both of its primary objectives: remission at week 26 and sustained remission at 52 weeks, as determined by a score of zero in the Birmingham Vasculitis Activity Score (BVAS) and being off glucocorticoid therapy for at least the previous four weeks.
Approximately 72.3% of avacopan-treated participants achieved disease remission at 26 weeks, compared with 70.1% of participants treated with standard glucocorticoid therapy.
This positive clinical response was sustained through 52 weeks in 65.7% of the avacopan-treated participants and in 54.9% in the glucocorticoid-treated group.
These long-term clinical data demonstrate that avacopan may be an alternative treatment for AAV that can lead to similar or even better outcomes than available standard-of-care steroid-based therapies — and can do so with lower toxicity.
Birmingham vasculitis activity score
CYCLOPS AND RELATED TRIALS :: Details of the trial design.
EULAR recommendation of primary small and medium vessel vasculitis. Conclusions: More specific items relate to starting immunosuppressive therapy in combination with glucocorticoids to induce remission, followed by a period of remission maintenance; for remission induction in life-threatening or organ-threatening AAV, cyclophosphamide and rituximab are considered to have similar efficacy; plasma exchange which is recommended, where licensed, in the setting of rapidly progressive renal failure or severe diffuse pulmonary hemorrhage.
PLASMAPHERESIS vs IV MP (MEPEX trial. EUVAS study group). Conclusion: Current approaches to treatment in induction and maintenance of AAV are well established (Table 3). The European League against Rheumatism (EULAR) guidelines for the management of small and medium vessel vasculitis have recently been published (ref) and the role of newer agents such as MMF is being defined by clinical trials. The success and safety profile of rituximab in refractory disease has led to trials in maintenance and induction therapy which may see it recommended as standard practice, although high cost may limit its use.
Among patients with severe ANCA-associated vasculitis, the use of plasma exchange did not reduce the incidence of death or ESKD. A reduced-dose regimen of glucocorticoids was noninferior to a standard-dose regimen with respect to death or ESKD. (Funded by the U.K. National Institute for Health Research and others; PEXIVAS Current Controlled Trials number, ISRCTN07757494; ClinicalTrials.gov number, NCT00987389.)
_______________________________________
A6-Anemia
NHCT trial. The Normal Hematocrit Cardiac Trial (NHCT) was the first large, randomized study of patients receiving hemodialysis to examine the outcomes of treating anemia to a target hematocrit range of 42 +/- 3% versus maintaining partial correction in a range of 30 +/- 3%. The results of the NHCT and a meta-analysis adding eight subsequent trials of normalization of hematocrit/hemoglobin in chronic kidney disease (CKD) have demonstrated increased thrombovascular events and mortality associated with the higher targets. .
Create study. In patients with chronic kidney disease, early complete correction of anemia does not reduce the risk of cardiovascular events.
CHOIR study. The use of a target hemoglobin level of 13.5 g per deciliter (as compared with 11.3 g per deciliter) was associated with increased risk and no incremental improvement in the quality of life.
Addendum: None of the randomized trials has reported an association between higher attained hemoglobin concentration and mortality within randomized groups. Mean platelet count did not increase among the patients in the normal-hematocrit group in the NHCT or in two other large trials, CREATE and CHOIR. Exposure to high doses of erythropoietic stimulating agents and/or intravenous iron could be mediating complications in the CKD anemia-normalization studies, but post-hoc analyses to probe such potential associations have yielded conflicting results and are clearly hindered by the risk of confounding by indication. The mechanisms underlying the deleterious outcomes associated with efforts to correct renal anemia fully remain unproven
TREAT study - Anemia and CVS mortality. The use of darbepoetin alfa in patients with diabetes, chronic kidney disease, and moderate anemia who were not undergoing dialysis did not reduce the risk of either of the two primary composite outcomes (either death or a cardiovascular event or death or a renal event) and was associated with an increased risk of stroke. For many persons involved in clinical decision making, this risk will outweigh the potential benefits.
PIVOTAL study on Anaemia in ESRD. Among patients undergoing hemodialysis, a high-dose intravenous iron regimen administered proactively was superior to a low-dose regimen administered reactively and resulted in lower doses of erythropoiesis-stimulating agent being administered.
Preemptive IV iron to maintain TS>30%/Ferritin>400, with target haemoglobin 10-12g/dl, reduces Epo related risks.
_______________________________________
A7-Hyperkalaemia
OPAL HK STUDY on Patiromer. (FDA approved 10/18) :: In patients with chronic kidney disease who were receiving RAAS inhibitors and who had hyperkalemia, patiromer treatment was associated with a decrease in serum potassium levels and, as compared with placebo, a reduction in the recurrence of hyperkalemia.
ZS-9 study. (Acute & chronic hyperkalemia). :: A significant reduction in potassium levels at 48 hours.
___________________________________
A8- Lupus nephritis
Lupus nephritis new treatment 2015: Treatment of severe lupus nephritis: the new horizon. Conclusion - This Review discusses the evidence in support of current standard of care immunosuppressive treatments and emerging therapies, and describes their roles and relative merits in the management of patients with lupus nephritis.
EURO-LUPUS study: High-dose IV CYC regimen (6 monthly pulses and 2 quarterly pulses; doses increased according to the white blood cell count nadir) or a low-dose IV CYC regimen (6 fortnightly pulses at a fixed dose of 500 mg), each of which was followed by AZA. Intent-to-treat analyses were performed. Conclusion - low-dose IV CYC (cumulative dose 3 gm) followed by AZA achieves clinical results comparable to those obtained with a high-dose regimen.
ALMS study: MMF did not show superiority over IVC for the induction therapy of LN, as measured by renal response rate after 24 wk of treatment. Interestingly, there was a statistically significant interaction between treatment group and race and between treatment group and region. Results did not differ between treatment groups for any secondary renal or nonrenal efficacy end points. However, there were more death in MMF group, more sepsis. Advantages were convenience and non-steility.
Systematic review and meta-analysis: There is currently insufficient evidence to determine which of the immunosuppressive agents is superior. The probability of renal remission is 50% or lower at 6 months.
Latest review of LN immune therapy (2016): They analyzed existing incidence and outcomes data, reviewed diagnosis and treatment guidelines, and evaluated the role rheumatologists play in the therapeutic process.
Asian lupus management review: Lupus nephritis (LN) is a common and severe organ involvement manifesting itself in systemic lupus erythematosus (SLE). There is a considerable difference in prevalence, severity, treatment response and outcomes between Asian LN patients and LN patients from other racial backgrounds.
Quote:"Two randomized placebo-controlled phase III trials demonstrated that belimumab (a B-lymphocyte stimulator antagonist), when added to standard treatment, could improve response rates in SLE patients without severe nephritis [68,69]. Post hoc analysis of patients with renal involvement from these trials suggested an efficacy signal of belimumab in LN, and thus further studies are underway [70]"
Belimumab trial on LN: A total of 448 patients underwent randomization (224 to the belimumab group and 224 to the placebo group). At week 104, significantly more patients in the belimumab group than in the placebo group had a primary efficacy renal response (43% vs. 32%; odds ratio, 1.6; 95% confidence interval [CI], 1.0 to 2.3; P=0.03) and a complete renal response (30% vs. 20%; odds ratio, 1.7; 95% CI, 1.1 to 2.7; P=0.02). The risk of a renalrelated event or death was lower among patients who received belimumab than among those who received placebo (hazard ratio, 0.51; 95% CI, 0.34 to 0.77; P=0.001). The safety profile of belimumab was consistent with that in previous trials.
CYCLOPS study 2009: Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis. The pulse cyclophosphamide regimen induced remission of ANCA-associated vasculitis as well as the daily oral regimen at a reduced cumulative cyclophosphamide dose and caused fewer cases of leukopenia.
CYCLOPS study - long-term outcomes: - long-term outcomes of patients of cyclops study. Pulse cyclophosphamide is associated with a higher relapse risk than daily oral cyclophosphamide. However, this is not associated with increased mortality or long-term morbidity. Although the study was retrospective, data was returned in 90% of patients from the original trial.
_______________________________________
A9- GN, IgAN,
a) MGN MENTOR study Rituximab and ciclosprin in primary MGN. Discussion: data and evaluation of pre- and posttreatment laboratory data. At the 6-month post-randomization visit, patients who have been randomized to either CSA or rituximab but who do not have a reduction in proteinuria ≥25% (confirmed on repeat measurements within 2 weeks) will be considered treatment failures and exit the study. receive intravenous rituximab (two infusions, 1000 mg each, administered 14 days apart; repeated at 6 months in case of partial response) or oral cyclosporine (starting at a dose of 3.5 mg per kilogram of body weight per day for 12 months
At 12 months, 39 of 65 patients (60%) in the rituximab group and 34 of 65 (52%) in the cyclosporine group had a complete or partial7
Serious adverse events occurred in 11 patients (17%) in the rituximab group and in 20 (31%) in the cyclosporine group (P=0.06
CONCLUSIONS
Rituximab was noninferior to cyclosporine in inducing complete or partial remission of proteinuria at 12 months and was superior in maintaining proteinuria remission up to 24 months.
b). MGN. STARMEN Trial in MGN The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy.
Hypothesis : A cyclical corticosteroid-cyclophosphamide regimen is recommended for patients with primary membranous nephropathy at high risk of progression. We hypothesized that sequential therapy with tacrolimus and rituximab is superior to cyclical alternating treatment with corticosteroids and cyclophosphamide in inducing persistent remission in these patients.
To receive six-month cyclical treatment with corticosteroid and cyclophosphamide or sequential treatment with tacrolimus (full-dose for six months and tapering for another three months) and rituximab (one gram at month six). The primary outcome was complete or partial remission of nephrotic syndrome at 24 months.
Concluded : treatment with corticosteroid-cyclophosphamide induced remission in a significantly greater number of patients with primary membranous nephropathy than tacrolimus-rituximab.
c) PPX. DFPP IN GN Comparison of double filtration plasmapheresis with immunoadsorption therapy in patients with anti-glomerular basement membrane nephritis. Conclusion- DPPP plus immunosuppressive therapy efficiently and safely removed anti-GBM antibodies. The fewer plasma-associated side effects and reduced loss of IgG suggest that DFPP may be a better treatment choice for anti-GBM disease, especially in patients with insufficient plasma.VALIGA study. European Validation Study of the Oxford Classification of IgAN.
d). IgAN TESTING study. Conclusions and Relevance
Among patients with IgA nephropathy and proteinuria of 1 g/d or greater, oral methylprednisolone was associated with an increased risk of serious adverse events, primarily infections. Although the results were consistent with potential renal benefit, definitive conclusions about treatment benefit cannot be made, owing to early termination of the trial.
STOP-IGAN study. The Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgA Nephropathy (STOP-IgAN) trial showed that after 3 years, full clinical remission had occurred in 5% of patients in the supportive-care group, as compared with 17% of patients who received immunosuppression with steroids plus cyclophosphamide followed by azathioprine.
IgAN VALIGA paper. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Summary:
The VALIGA study provides a validation of the Oxford classification in a large European cohort of IgAN patients across the whole spectrum of the disease. The independent predictive value of pathology MEST score is reduced by glucocorticoid/ immunosuppressive therapy.
POS-830 NEFECON FOR THE TREATMENT OF IgA NEPHROPATHY IN PATIENTS AT RISK OF PROGRESSING TO END-STAGE RENAL DISEASE: THE NEFIGARD PHASE 3 TRIAL RESULTS
NefIgArd is a randomized, double-blind, placebo-controlled clinical trial recruiting a total of 360 patients across 155 nephrology clinics in 20 countries.
NEFECON group provided a statistically significant 7% (3.87 mL/min/1.73m2) treatment benefit on eGFR (p=0.0029), compared with placebo. There was a similar overall incidence of adverse events in the two treatment groups.
The NefIgArd study met its primary endpoint and demonstrated a favorable safety profile. These results are indicative of a clinically meaningful reduced risk of future progression to end-stage renal disease in patients with IgAN treated with NEFECON.
In the NEFIGARD trial (ClinicalTrials.gov Identifier: NCT03643965), patients are randomized to placebo or enteric release budesonide (Nefecon®) based on a phase II RCT, which demonstrated that this enteric corticosteroid reduced proteinuria and eGFR loss over 1 year in IgAN [73].
In the ARTEMIS trial (ClinicalTrials.gov Identifier: NCT03608033), patients are randomized to placebo or repeated infusions of an antibody to MASP-2, the key enzyme regulating the activity of the mannose-binding lectin pathway of complement. In a small phase II RCT, this antibody had markedly reduced proteinuria in IgAN patients [74].
There are more phase II or III clinical trials to evaluate the efficacy and safety of inhibiting other arms of the complement cascade in IgAN, for example, (i) alternative pathway—using LNP023, an orally available, small-molecule inhibitor of complement factor B (APPLAUSE trial; ClinicalTrials.gov Identifier: NCT04578834) in which appropriate prior vaccinations against pneumococci, meningococci, and haemophilus influenzae B are needed and (ii) terminal converging pathway—using ravulizumab, a C5 inhibitor (ClinicalTrials.gov Identifier: NCT04564339).
Yet, another non-immunosuppressive combination therapy approach gained attention in IgAN and other glomerular diseases over the last years, i.e., a new-class drug of a dual acting ARB and endothelin receptor antagonist (ERA) named sparsentan. Preclinical studies in animal models demonstrated that ERAs may reduce proteinuria by ameliorating kidney damage and in subsequent trials a combined ERA/ARB therapy exerted additive antiproteinuric effects in patients with glomerular diseases such as diabetic nephropathy, FSGS, and IgA nephropathy [75–77]. As such, ERAs emerge as a highly promising novel treatment principle that may even augment effects of a single RASB therapy:
In the phase III PROTECT trial (ClinicalTrials.gov Identifier: NCT03762850), patients are randomized to receive irbesartan or sparsentan, a dual angiotensin-II and endothelin-1 receptor blocker, based on a phase II trial in patients with focal segmental glomerulosclerosis [77].
Similar to PROTECT, another RCT (ALIGN; ClinicalTrials.gov Identifier: NCT04573478) also targets endothelin-1 using the specific endothelin-A receptor blocker atrasentan.
_______________________________________
A10-DM outcome trial
DCCT & EDIC Studies. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 Years: Overview. CONCLUSIONS: DCCT/EDIC has demonstrated the effectiveness of INT in reducing the long-term complications of T1DM and improving the prospects for a healthy life span.
UKPDS Study. The UK Prospective Diabetes Study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Paromita King, Ian Peacock, and Richard Donnelly. Conclusion: The UKPDS provides management guidelines for selected patients, but leaves many questions unanswered. The advantages of good care have been more clearly defined than ever before, but the huge gulf between the benefits achieved in the study and the many frustrations of everyday practice remains. In most centres, there are large numbers of patients with poor control of both blood glucose and blood pressure. Type 2 diabetes must at least be taken more seriously.
UKPDS 10 years followup. CONCLUSIONS: Despite an early loss of glycemic differences, a continued reduction in microvascular risk and emergent risk reductions for myocardial infarction and death from any cause were observed during 10 years of post-trial follow-up. A continued benefit after metformin therapy was evident among overweight patients.
NEPHRON-D study, Combination therapy with an ACE inhibitor and an ARB was associated with an increased risk of adverse events among patients with diabetic nephropathy.
DERIVE study. The DERIVE trial randomized 321 patients with T2D (hemoglobin A1C [HbA1C] between 7-11%; mean 8.2%) and stage 3A CKD (mean estimated glomerular filtration rate [eGFR] 53 mL/min/1.73m2) from eight countries and treated them with either dapagliflozin 10 mg or placebo over 24 weeks. Mean eGFR was decreased at 24 weeks with dapagliflozin (-3.23 mL/min/1.73m2) vs placebo.
SGLT-2 inhibitors, positive outcome in DN CKD. Conclusion: Currently available data suggest that, despite only modest reductions in glycated haemoglobin, SGLT2 inhibitors reduce the risk of cardiovascular and renal outcomes in patients with T2DM and CKD, without clear evidence of additional safety concerns.
CREDENCE TRIAL: Canagliflozin and Renal Outcomes in Type 2 Diabetes & Nephropathy. CONCLUSION: in patients with type 2 diabetes and kidney disease, the risk of kidney failure and cardiovascular events was lower in the canagliflozin group than in the placebo group at a median follow-up of 2.62 years.
PACE SLIDES ON SGLT-2 (Section C\4).
ADVANCE Trial (Action in Diabetes and Vascular disease: preterax and diamicron MR controlled evaluation) . Conclusions: A strategy of intensive glucose control, involving gliclazide (modified release) and other drugs as required, that lowered the glycated hemoglobin value to 6.5% yielded a 10% relative reduction in the combined outcome of major macrovascular and microvascular events, primarily as a consequence of a 21% relative reduction in nephropathy.
ACCORD trial (Action to Control Cardiovascular Risk in Diabetes): CONCLUSIONS - As compared with standard therapy, the use of intensive therapy to target normal glycated hemoglobin levels for 3.5 years increased mortality and did not significantly reduce major cardiovascular events. These findings identify a previously unrecognized harm of intensive glucose lowering in high-risk patients with type 2 diabetes.
PRESERVE STUDY on Contrast nephropathy Contrast-Induced Acute Kidney Injury in the PRESERVE Trial. The trial was stopped after a prespecified interim analysis indicated a low likelihood of seeing a meaningful difference in the primary end point, which was experienced by 4.7% in the saline group versus 4.4% in the bicarbonate group (P=0.62). Similarly, no differences were observed between patients receiving acetylcysteine and placebo (4.6% versus 4.5%, respectively; P=0.88). Lastly, no differences were observed in the rates of AKI, cardiovascular, or all-cause hospitalizations or adverse events, and there were no differences within key prespecified subgroups (including stratification by baseline eGFR, albuminuria, and contrast volume).
_______________________________________
A11- Hypertension
AASK trial - tight control of hypertension. CONCLUSIONS: In overall analyses, intensive blood-pressure control had no effect on kidney disease progression. However, there may be differential effects of intensive blood-pressure control in patients with and those without baseline proteinuria.
ABCD (absolute blood pressure control) trial. In both the hypertensive and normotensive studies, mean renal function (as assessed by 24 h creatinine clearance) remained stable during 5 years of either intensive or standard blood pressure intervention in patients with normoalbuminuria (<30 mg/24 h) or microalbuminuria (30-300 mg/24 h) at baseline. By contrast, the rate of creatinine clearance in patients with overt diabetic nephropathy (>300 mg/24 h; albuminuria) at baseline decreased by an average of 5 ml/min/year in spite of either intensive or standard blood pressure control. Analysis of the results of 5 years of follow-up revealed a highly significant correlation of all-cause and cardiovascular mortality with left ventricular mass and severity of albuminuria.
ACCORD. (Action to Control Cardiovascular Risk in Diabetes): Hypertension arm: CONCLUSIONS
In patients with type 2 diabetes at high risk for cardiovascular events, targeting a systolic blood pressure of less than 120 mm Hg, as compared with less than 140 mm Hg, did not reduce the rate of a composite outcome of fatal and nonfatal major cardiovascular events.
Framingham heart study. Summary: This is a historical review of the contribution of the Framingham Heart Study to our understanding of the epidemiology of blood pressure (BP) and cardiovascular disease (CVD). Framingham investigators initially explored the epidemiological relationship of various BP components to coronary heart diseases in men and women and how this risk is further modified by age, that is, how diastolic blood pressure (DBP) is the stronger predictor of coronary heart disease risk in young people versus systolic blood pressure (SBP) in middle-aged and elderly people. Framingham investigators then examined the natural history of various BP components over a 30-year follow-up in normotensive and untreated hypertensive individuals and showed how this provides hemodynamica insights into the importance of pulse pressure as a marker of large artery stiffness in middle-aged and elderly people. Importantly, pulse pressure was also found to be superior to SBP or DBP as a predictor of coronary heart disease in a middle-aged and elderly Framingham population. Lastly, dual models of SBP with DBP and pulse pressure and mean arterial pressure were superior to single BP component models for predicting CVD events; thus, increases in both periferal vascular resistance and central large artery stiffness contribute to CVD in varying proportions depending on age. Furthermore, the Framingham Heart Study provided evidence that DBP <70 mm Hg with SBP ≥120 mm Hg was associated with a CVD risk equivalent to approximately 20 mm Hg of additional elevation in SBP, thus further supporting the importance of large artery stiffness as a CVD risk factor in elderly people. These original Framingham studies have contributed greatly to BP risk classification tables for the “Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure” and for the European Society for Hypertension. Moreover, Framingham originally brought attention to hypertension, which is now the leading cause of mortality globally.
ON-TARGET study. Telmisartan, Ramipril, or Both in Patients at High Risk for Vascular Events. CONCLUSIONS: Telmisartan was equivalent to ramipril in patients with vascular disease or high-risk diabetes and was associated with less angioedema. The combination of the two drugs was associated with more adverse events without an increase in benefit.
ALTITUDE study on Aliskerin. Cardiorenal End Points in a Trial of Aliskiren for Type 2 Diabetes: CONCLUSIONS The addition of aliskiren to standard therapy with renin–angiotensin system blockade in patients with type 2 diabetes who are at high risk for cardiovascular and renal events is not supported by these data and may even be harmful.
Aliskerin, AVOID, ALTITUDE, APOLLO, ATMOSPHERE studies (section B\1).
SPRINT trial, intensive vs standard BP control. A Randomized Trial of Intensive versus Standard Blood-Pressure ControlList of authors.The SPRINT Research Group*. CONCLUSIONS: Among patients at high risk for cardiovascular events but without diabetes, targeting a systolic blood pressure of less than 120 mm Hg, as compared with less than 140 mm Hg, resulted in lower rates of fatal and nonfatal major cardiovascular events and death from any cause, although significantly higher rates of some adverse events were observed in the intensive-treatment group.
RCT on Spironolactone PATHWAY-2 study. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. InterpretationSpironolactone was the most effective add-on drug for the treatment of resistant hypertension. The superiority of spironolactone supports a primary role of sodium retention in this condition.
Symplicity HTN-2 trial, sympathetic denervation. :: Renal sympathetic denervation in patients with treatment-resistant, a randomised controlled trial.
_______________________________________
A12-Haemodialysis
HEMO Study: Effect of Dialysis Dose and Membrane Flux in Maintenance Hemodialysis. Conclusion - Patients undergoing hemodialysis thrice weekly appear to have no major benefit from a higher dialysis dose than that recommended by current U.S. guidelines or from the use of a high-flux membrane.
OL-HDF Study. HDF vs HD: Online-haemodiafiltration vs. conventional haemodialysis: a cross-over study. Conclusion - Comparing HDF and HD, we did not observe any differences in haemodynamic stability or in serum phosphate levels. Only serum ß2-m (−6 % vs. HD) and albumin (−5 % vs. HD) levels changed. The long-term clinical consequences of these biochemical differences should be prospectively assessed.
ESHOL study. HDF & SURVIVAL: High-efficiency postdilution online hemodiafiltration. Conclusion - Compared with patients who continued on hemodialysis, those assigned to OL-HDF had a 30% lower risk of all-cause mortality (hazard ratio [HR], 0.70; 95% confidence interval [95% CI], 0.53-0.92; P=0.01), a 33% lower risk of cardiovascular mortality (HR, 0.67; 95% CI, 0.44-1.02; P=0.06), and a 55% lower risk of infection-related mortality (HR, 0.45; 95% CI, 0.21-0.96; P=0.03). The estimated number needed to treat suggested that switching eight patients from hemodialysis to OL-HDF may prevent one annual death. The incidence rates of dialysis sessions complicated by hypotension and of all-cause hospitalization were lower in patients assigned to OL-HDF. In conclusion, high-efficiency postdilution OL-HDF reduces all-cause mortality compared with conventional hemodialysis.
IDEAL study: A Randomized, Controlled Trial of Early versus Late Initiation of Dialysis. Conclusion - In this study, planned early initiation of dialysis in patients with stage V chronic kidney disease was not associated with an improvement in survival or clinical outcomes.
_______________________________________
A13. Peritoneal dialysis
CANUSA study: (1996) Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Conclusion: A decrease of 0.1 unit Kt/V per week was associated with a 5% increase in the RR of death; a decrease of 5 L/1.73 m2 creatinine clearance (CCr) per week was associated with a 7% increase in the RR of death. The RR of technique failure was increased with decreased albumin concentration and decreased CCr. Hospitalization was increased with decreased serum albumin concentration, worsened nutrition according to subjective global assessment and decreased CCr. A weekly Kt/V of 2.1 and a weekly CCr of 70 L/1.73 m2 were each associated with an expected 2-yr survival of 78%.
The study concluded that it would appear reasonable to provide as high a dialysis dose as is feasible with a Kt/V of 2. 1 or a CCr of 70 L/week.
CANUSA (Re-analysis, 2001)
CANUSA data was reanalyzed to address the assumption that renal and peritoneal clearances are comparable and therefore additive. The findings were again remarkable. A 5-L greater weekly GFR was associated with a 12% decrease in the RR of death
But…It found no association between the same increase in peritoneal creatinine clearance and the RR of death. In fact, for each increase of 250 ml of urine per day, there was a 36% decrease in the RR of death. Thus reflecting the importance of residual renal function, and questioning the utility of targeting higher Kt/V.
ADEquacy of PD in MEXico, ADEMEX (2002)
This was a randomized control trial (RCT) which enrolled 965 patients in 1998-1999 and followed them for 2 years with death as the primary end point. The control received 4 daily exchanges of 2 L vs the intervention group which received multiple 2.5L to 3L exchanges to achieve a peritoneal creatinine clearance (pCrCl) value of 60 L/wk per 1.73 m2. It essentially compared 47 vs 60 L/wk/1.73 m2 of creatinine clearance or Kt/V of approximately 1.7 vs 2.0 per week. Importantly, residual renal function was similar in 2 groups.
They found that patient survival was similar for the control and intervention groups. Age, diabetes mellitus, serum albumin levels, residual renal function were all identified as significant factors associated with patient survival.
Hong Kong Study (2003)
Lastly, this RCT enrolled 320 new CAPD patients across 6 centers in Hong Kong with baseline renal Kt/V < 1.0, mean follow up period was 24.3 months. They were randomized to three Kt/V targets:
Group A: 1.5 to 1.7; Group B: 1.7 to 2.0; Group C: >2.0
They found that the total Kt/V did not significantly affect survival. There was also no difference in serum albumin, composite nutritional index (CNI) scores, and hospitalization rate. However, in Group A, a significantly higher number of patients were withdrawn from the study mainly due to inadequate ultrafiltration and clinical inadequacy of dialysis and more group A patients required EPO, possibly suggestive of uremia.
BALANZ TRIAL: Rationale and design of the balANZ trial: A randomised controlled trial of low GDP, neutral pH versus standard peritoneal dialysis solution for the preservation of residual renal function. INTERPRETATION: It is not the first study to report preservation of residual renal function with a biocompatible solution, and although the mechanism has not been worked out, possibilities could include a direct nephrotoxic effect of GDPs or a difference in volume status related to an early reduction in UF or, alternatively, to a reduced peritonitis rate.
PD HD mortality comparison: Comparison of Mortality between Japanese Peritoneal Dialysis and Hemodialysis Patients: A 5-Year Multicenter Follow-Up Study. Conclusion - This study showed that the overall mortality was not significantly different between PD and HD patients, which suggests that dialysis modality might not be an independent factor for survival in Japanese patients.
_______________________________________
A14-Transplantation
INTAC trial Alemtuzumab induction in renal transplant. CONCLUSIONS: By the first year after transplantation, biopsy-confirmed acute rejection was less frequent with alemtuzumab than with conventional therapy. The apparent superiority of alemtuzumab with respect to early biopsy-confirmed acute rejection was restricted to patients at low risk for transplant rejection; among high-risk patients, alemtuzumab and rabbit antithymocyte globulin had similar efficacy. (Funded by Astellas Pharma Global Development; INTAC ClinicalTrials.gov number, NCT00113269.).
3C trial Alemtuzumab-based induction treatment versus basiliximab-based induction treatment in kidney transplantation (the 3C Study): a randomised trialThe 3C Study Collaborative Group. Conclusion: Compared with standard basiliximab-based treatment, alemtuzumab-based induction therapy followed by reduced CNI and mycophenolate exposure and steroid avoidance reduced the risk of biopsy-proven acute rejection in a broad range of patients receiving a kidney transplant. Long-term follow-up of this trial will assess whether these effects translate into differences in long-term transplant function and survival.
BENEFIT trial A Phase III Study of Belatacept‐based Immunosuppression Regimens versus Cyclosporine in Renal Transplant Recipients. Abstract: Belatacept, a costimulation blocker, may preserve renal function and improve long‐term outcomes versus calcineurin inhibitors in kidney transplantation: Safety was generally similar between groups, but posttransplant lymphoproliferative disorder was more common in the belatacept groups. CONCLUSIONS
Seven years after transplantation, patient and graft survival and the mean eGFR were significantly higher with belatacept (both the more-intensive regimen and the less-intensive regimen) than with cyclosporine. (
CONVERT trial: Lower malignancy rates in renal allograft recipients converted to sirolimus-based, calcineurin inhibitor-free immunotherapy: 24-month trial.
Symphony trial (Elite-Symphony). Standard vs low dose CNI. A regimen of daclizumab, mycophenolate mofetil, and corticosteroids in combination with low-dose tacrolimus may be advantageous for renal function, allograft survival, and acute rejection rates, as compared with standard regimens.
Immunosuppressive regimens with the fewest possible toxic effects are desirable. This study evaluated the efficacy and relative toxic effects of four immunosuppressive regimens. 1645 renal-transplant recipients to receive standard-dose cyclosporine, mycophenolate mofetil, and corticosteroids, or daclizumab induction, mycophenolate mofetil, and corticosteroids in combination with low-dose cyclosporine, low-dose tacrolimus. The primary end point was the estimated glomerular filtration rate (CCG-GFR). Secondary end points included acute rejection and allograft survival. GFR was higher in patients receiving low-dose tacrolimus. The rate of biopsy-proven acute rejection was lower in patients receiving [Study arm] low-dose tacrolimus (12.3%) than in those receiving (1) standard-dose cyclosporine (25.8%), (2) low-dose cyclosporine (24.0%), or (3) low-dose sirolimus (37.2%). Allograft survival differed significantly among the four groups (P=0.02) and was highest in the low-dose tacrolimus group (94.2%), followed by the low-dose cyclosporine group (93.1%), the standard-dose cyclosporine group (89.3%), and the low-dose sirolimus group (89.3%). Serious adverse events were more common in the low-dose sirolimus group than in the other groups (53.2% vs. a range of 43.4 to 44.3%), although a similar proportion of patients in each group had at least one adverse event during treatment (86.3 to 90.5%). A regimen of daclizumab, mycophenolate mofetil, and corticosteroids in combination with low-dose tacrolimus may be advantageous for renal function, allograft survival, and acute rejection rates, as compared with regimens containing daclizumab induction plus either low-dose cyclosporine or low-dose sirolimus or with standard-dose cyclosporine without induction.
FK vs CsA: Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. Conclusions: Treating 100 recipients with tacrolimus instead of ciclosporin for the first year after transplantation avoids 12 patients having acute rejection and two losing their graft but causes an extra five patients to develop insulin dependent diabetes. Optimal drug choice may vary between patients.
PRE-DIABETES: Prediabetes in patients receiving tacrolimus in the first year after kidney transplantation: a prospective and multicenter study. CONCLUSION: One in two recipients with tacrolimus-based immunosuppresion showed prediabetes or NODAT by 1 year posttransplantation when properly investigated. Older age and high pretransplant body mass index and triglyceride/high density lipoprotein-cholesterol ratio were risk factors for prediabetes. These findings may help applying early interventions to prevent the disorder.
STEROID WITHDRAWAL Steroid Avoidance or Withdrawal After Renal Transplantation Increases the Risk of Acute Rejection but Decreases Cardiovascular Risk. A Meta-analysis.
MMF IN AR: Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review.
MALIGNANCY IN TRANSPLANT. Registry data show that there is an overall 3–5-fold increase in cancer risk in transplant recipients compared with the general population, with skin cancers and lymphoma particularly prevalent. Cancers in transplant recipients are often more aggressive than those in the general population, with poor prognosis, particularly for gastrointestinal tumours and lymphomas.
TRANSFORM STUDY. Conclusion- the EVR + rCNI regimen offers comparable efficacy and graft function with low tBPAR and dnDSA rates and significantly lower incidence of viral infections relative to standard‐of‐care up to 24 months.
INFINITY trial. Three-Year Outcomes in Kidney Transplant Patients Randomized to Steroid-Free Immunosuppression or Steroid Withdrawal, with Enteric-Coated Mycophenolate Sodium and Cyclosporine: The Infinity Study. Conclusion- IL-2RA induction with early intensified EC-MPS dosing and CNI therapy in de novokidney transplant patients at low immunological risk may achieve similar three-year efficacy regardless of whether oral steroids are withheld for at least three months.
IMPACT study. Impact of patterns of proteinuria on renal allograft function and survival: a prospective cohort study. Conclusion: Proteinuria in renal transplants can be differentiated into glomerular and tubular types based on molecular weight. Glomerular proteinuria is associated with significant increase in graft dysfunction and graft loss.
Urinary proteomic. Significance of Urinary Proteome Pattern in Renal Allograft Recipients. Abstract: Urinary proteomics is developing as a platform of urinary biomarkers of immense potential in recent years. The definition of urinary proteome in the context of renal allograft and characterization of different proteome patterns in various graft dysfunctions have led to the development of a distinct science of this noninvasive tool. Substantial numbers of studies have shown that different renal allograft disease states, both acute and chronic, could portray unique urinary proteome pattern enabling early diagnosis of graft dysfunction.
_______________________________________
A15. MBD, URIC Acid
CARE Study on sevelamer - Treatment of hyperphosphatemia in hemodialysis patients: The Calcium Acetate Renagel Evaluation (CARE Study). CONCLUSION: Calcium acetate controls serum phosphorus and calcium-phosphate product more effectively than sevelamer hydrochloride. Cost-benefit analysis indicates that in the absence of hypercalcemia, calcium acetate should remain the treatment of choice for hyperphosphatemia in hemodialysis patients.
CARE-2 Study 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Conclusion: With intensive lowering of LDL-C levels for 1 year, hemodialysis patients treated with either calcium acetate or sevelamer experienced similar progression of CAC.
The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. In hemodialysis patients with moderate to severe secondary hyperparathyroidism, cinacalcet plus low-dose vitamin D sterols may attenuate vascular and cardiac valve calcification.
ECHO study on Cinacalcet - Effectiveness of cinacalcet in patients with recurrent/persistent secondary hyperparathyroidism following parathyroidectomy: results of the ECHO study. CONCLUSIONS: Our data support the successful use of cinacalcet in patients with recurrent/persistent sHPT after PTX.
IMPACT Study - Paricalcitol versus cinacalcet plus low-dose vitamin D therapy for the treatment of secondary hyperparathyroidism in patients receiving hemodialysis: results of the IMPACT SHPT study. CONCLUSION: Paricalcitol versus cinacalcet plus low-dose vitamin D provided superior control of iPTH, with low incidence of hypercalcaemia.
CARES study on Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout. Conclusion: In patients with gout and major cardiovascular coexisting conditions, febuxostat was noninferior to allopurinol with respect to rates of adverse cardiovascular events. All-cause mortality and cardiovascular mortality were higher with febuxostat than with allopurinol.
FAST Study- interim analysis in European population shows no difference in cardiovascular mortality in CKD as compared to Allopurinol.
_______________________________
____________________________________
Section B: RCT Critical Appraisal
B1. Lupus nephritis (Glassok)
B2. ADPKD (Mark)
B3. ANCA Vasculitis (Khawja)
B4. AKI (Levin)
B5. Primary GN (Glassok)
B6. CKD (Nahas)
B7. Anaemia (Kossi)
B8. Lipid (Kossi)
B9. DN (Meguud)
B10.MBD (Mohsen)
B11. Haemodialysis (Charles)
B12. Peritoneal dialysis (Davies)
_______________________________________
B1. Lupus nephritis (Glassock)
The interest in finding effective and safe treatments for lupus nephritis (LN) has been both long standing and intense [1]. Achieving this goal has been very frustrating since no new treatment has been approved by regulatory authorities for the treatment of LN in over 60 years. Nevertheless, many randomized clinical trials have been designed and executed, and some have been instrumental in altering the landscape of “off-label” management of LN, particularly in severe proliferative forms. It is generally believed that combinations of high-dose anti-inflammatory glucocorticoids and some type of immunosuppressive agents are required for adequate control of LN that will delay or prevent its progression to ESRD and ameliorate the consequences of marked proteinuria (nephrotic syndrome) while at the same time reducing the burden of extrarenal manifestations of systemic lupus erythematosus (e.g., arthritis, dermatitis, serositis, and hematological/neurological disturbances). But LN is in all likelihood not due to a single pathogenic process, and it can present with diverse clinical and pathological features. Relapses of clinically active disease are common and difficult to predict. The value of intermediate outcomes (surrogates) for prediction of hard endpoints (such as ESRD) has been difficult to prove, since the occurrence of these endpoints is often delayed by 5 or more years from the apparent onset of disease, with contemporary regimens. Adverse events consequent to treatment are common and occasionally fatal, further confounding the execution and interpretation of studies. This heterogeneity, unpredictability, and susceptibility to complicating diseases has hampered the conduct of LN randomized clinical trials. Finding suitable surrogate endpoints for trial design has been a challenge [2]. Nevertheless, progress has occurred and several treatment regimens are now generally regarded as effective and reasonably safe for management of severe proliferative LN. The situation for treatment of the pure membranous LN is much less certain [3].
Ref:https://link.springer.com/chapter/10.1007/978-3-319-10292-4_2
_______________________________________
B2. ADPKD (Mark)
Autosomal dominant polycystic kidney disease (ADPKD) is the most common monogenic hereditary kidney disease in humans, with a prevalence of 1 out of every 800–1,000 individuals, and is the cause of end-stage renal disease (ESRD) in 5–10 % of the prevalent patients on renal replacement therapy (RRT) worldwide [1]. The disease is characterized by the development, growth, and expansion of multiple renal cysts, leading to destruction of normal renal parenchyma, massively enlarged kidneys, and subsequent kidney function loss [2–4]. The natural course of ADPKD is often of progressive nature, eventually leading to ESRD in approximately 50 % of patients afflicted.
Ref:https://link.springer.com/chapter/10.1007/978-3-319-10292-4_1
_______________________________________
B3. ANCA ASSOCIATED Vasculitis (Khawja)
Antineutrophil cytoplasmic antibodies (ANCA) were first described in 1982 in patients with pauci-immune glomerulonephritis. Since then, circulating ANCA has been recognised in a range of small vessel vasculitides collectively as ANCA-associated vasculitis (AAV). AAV encompasses a number of clinical syndromes including granulomatosis with polyangitis (GPA formerly known as Wegener’s granulomatosis), microscopic polyangitis (MPA) and Churg-Strauss (CSS). Proteinase 3 (PR3)-ANCA is strongly associated with patients with GPA, whilst myeloperoxidase (MPO)-ANCA occurs more frequently in MPA.
Ref: https://link.springer.com/chapter/10.1007/978-3-319-10292-4_3
_______________________________________
B4. AKI (Levin)
Clinical trials in the area of acute kidney injury (AKI) over the last decade have focused on attenuating the incidence of AKI (contrast nephropathy), managing AKI (diuretics, dopamine), or improving the outcomes of established AKI and kidney failure (intensity or type of dialysis). As a community we have struggled with matching our understanding of the physiology of kidney failure with testing hypotheses within complex clinical conditions using robust clinical studies to determine the best care for patients with AKI. The current limitations of our knowledge are a function of the difficulty of executing large-scale clinical trials in this area given the complexity of the patient population. This chapter highlights key studies that examine the role of N-acetylcysteine, hydration, and type of contrast agent on the incidence, severity, and duration of contrast nephropathy; the role of diuretics and dopamine on duration and severity of AKI; the utility of different methods of fluid removal in acute decompensated heart failure (ultrafiltration vs. diuretics); and the impact of different dialysis prescriptions or methods on patient outcomes. All of the studies selected reflect important questions, relevant in clinical practice. Due to limited ability to truly diagnose AKI, we may not be able to intervene in a timely manner, and our therapies, once kidney failure is established, are surely predicated on the etiology and duration of the AKI insult. Nonetheless, the studies described herein reflect the best available data to guide therapy and suggest that IV hydration to approximately 1 l is of benefit in attenuation of the onset of AKI in a number of high-risk situations, that bolus vs. continuous infusions of loop diuretics are not substantially different, that low-dose dopamine does not improve kidney outcomes, that intensity of CRRT may not impact long-term outcomes, and that PD in acute situations may be a viable renal replacement method. Some of the studies are small (<500 patients) and all have their limitations. They represent our best understanding at the current time. In the coming decade, we need more robust, inclusive, and large-scale studies which address other important questions such as best way to diagnosis early AKI, appropriate early interventions to avoid dialysis, and timing of dialytic intervention in AKI. Of additional note is the problem of defining hard outcomes in AKI. While need for dialysis or death are certainly hard outcomes, the importance of small changes in serum creatinine cannot be overestimated. In a numerous clinical and administrative databases, any change in serum creatinine above 26 μmol/l is associated with increased risk of CKD, CVD, hospitalizations, and death. Thus, we have chosen in this series to “accept” a change in serum creatinine as reasonable “hard end point” while acknowledging that not all would agree. In order to acknowledge the problems with a change in biomarker being a primary end point, we have altered the scoring system for this parameter in the tables below. In the meantime, we would hope that the results of these trials could be incorporated into clinical practice.
Ref:link.springer.com/chapter/10.1007/978-3-319-10292-4_4
_______________________________________
B5. Primary GN (Glassock)
The evidence base upon which a rational approach to the management of primary glomerular disease has been building slowly over many decades. Unfortunately, the quality of the studies encompassing this database has varied widely, in part due to difficulties in study design and subject enrolment. Many studies have been small and underpowered and have used surrogate endpoints, such as a change in proteinuria. Unintentional or unavoidable biases have crept into study execution and few have been sufficiently long term to evaluate “hard” outcomes such as avoidance of end-stage renal disease (ESRD). The use of estimated rather than measured GFR may have added a further bias in the few studies with renal function endpoints. Due to the limited knowledge of etiology and pathogenesis of primary glomerular disease, most studies have examined the effects of “non-specific” empirical treatments (such as the use of glucocorticoids or immunosuppressive agents). Nevertheless, there is a “ray of sunshine”. Recent trials have employed more rigorous trial design and sample sizes appropriate to minimize the β-error (false negatives). Increasingly, the focus has been on more homogeneous group of enrolees with a reasonably well-defined prognosis, in the absence of treatment. Active comparator rather than placebo-controlled trials dominate the landscape and many trials are not double-blinded leading to the potential for bias.
Ref:https://link.springer.com/chapter/10.1007/978-3-319-10292-4_10
_______________________________________
B6. CKD (Nahas)
For the last three decades, and since the publication of the “Hyperperfusion–Hyperfiltration” hypothesis by Brenner and his colleagues in Boston, USA [1], considerable research has focused on the understanding of the pathophysiology of chronic kidney disease (CKD). Numerous additional hypotheses and theories have been published, focusing on the key role of renal as well as extrarenal cells in the pathogenesis of progressive renal scarring and fibrosis and the consequent decline in kidney function witnessed in CKD. These have been followed by a slow transition from the preclinical world of laboratory investigations to the bedside with a number of key clinical trials.
Ref:https://link.springer.com/chapter/10.1007/978-3-319-10292-4_5
_______________________________________
B7. Anemia in CKD (Kossi)
The pivotal role of haemoglobin as one of the targets in the holistic management of dialysis patients was the focus of the first published anaemia guidelines by KDOQI in 1997. Since the introduction of erythropoietin to anaemia management in chronic kidney disease, there has been a continuous debate on the optimal haemoglobin target required to achieve the desirable clinical outcome whether in survival or quality of life. With the unequivocal evidence of the impact of these agents on relatively better quality of life and reduced need for red-cell transfusion with the anticipated potential complications attached to it, there is an element of uncertainty about the exact risk of these agents on chronic kidney disease patients. Iron deficiency is the other area that attracts a lot of interest in anaemia management in chronic kidney disease. Absolute and relative iron deficiencies are recognised for a long time as important contributing factors for anaemia development in different stages of chronic kidney disease. More importantly, response to erythropoiesis stimulating agents (ESAs) is suboptimal in the presence of iron deficiency. Most of the up-to-date anaemia guidelines emphasise on the central role of iron in anaemia management. Disappointing enough, iron treatment recommendations are either not graded or scored low. This emphasises the unmet need for better quality evidence to address a confusing dilemma in iron treatment in this group of patients. Disproportionately, a large number of high-quality and well-designed studies became available in ESA treatment area which allowed for high-rank quality of evidence. Questions that remain without clear answers are: When to start iron treatment? Which route of administration to use? What are the targets of iron treatment? What are the best biochemical variables that reflect iron status? A very limited number of reasonable quality studies have tried to answer some of these questions. I included some of those trials that attracted a lot of debate in this area and have remarkable impact on anaemia management in chronic kidney disease patients whether on dialysis or not. I also randomly included some of the landmark ESA trials at different levels of chronic kidney disease.
Ref: https://link.springer.com/chapter/10.1007/978-3-319-10292-4_6
_______________________________________
B8. Lipid in CKD (Kossi)
Cardiovascular disease is the leading cause of mortality and morbidity in patients with different stages of chronic kidney disease (CKD). The phenotypic picture of cardiovascular disease differs between those on dialysis and patients with early stages of chronic kidney disease. Whilst atherosclerotic coronary artery disease is common in the early stages of CKD, arrhythmias, congestive cardiac failure and sudden cardiac death (SCD) prevail in ESRD patients on dialysis, thus confounding interventions. Lipids profile also differs between both stages with high cholesterol levels being common at early CKD stages with relatively well-nourished patients compared to normal or low cholesterol levels in ESRD patients treated by dialysis who are usually under- or malnourished; reverse epidemiology. Lipid profiles also differ between haemodialysis and peritoneal dialysis patients; due to the high caloric content of peritoneal dialysis fluids, patient treated by this modality have higher triglycerides levels.
Ref:://link.springer.com/chapter/10.1007/978-3-319-10292-4_7
_______________________________________
B9. DN (Meguid)
Over the last quarter of a century, diabetic nephropathy has steadily increased in incidence and prevalence to become one of the most common causes of ESRD. Worldwide, obesity and the associated type 2 diabetes mellitus (T2DM) have reached epidemic proportions leading to a significant rise in those suffering from diabetic kidney disease (DKD) [1]. This has coincided with considerable preclinical research aimed at a better understanding of the pathophysiology of diabetic nephropathy (DN) and its progression [2].
Ref:https://link.springer.com/chapter/10.1007/978-3-319-10292-4_9
_______________________________________
B10. MBD (Mohsen)
Chronic Kidney Disease-Mineral Bone Disorder (CKD-MBD) refers to a triad of mineral, bone and vascular disorders that are associated with an increased risk of morbidity and mortality in CKD. The last 15 years have seen a huge increase in research activity in this field as a result of better understanding of pathogenesis of bone disease and its inter-relationship with cardiovascular disease. Furthermore epidemiological data indicate that disturbances in mineral metabolism have been associated with an increased risk of vascular calcification, left ventricular hypertrophy, cardiovascular mortality as well as an increased risk of fractures [1]. It is clear that the pathogenesis of bone and vascular disease is not simply dependent on calcium, phosphorus, vitamin D and parathyroid hormone (PTH) but a host of other factors, most notably the fibroblast growth factor 23 (FGF23)-Klotho axis [2]. FGF23 acts as a potent phosphatonin increasing the expression of the sodium-phosphate co-transporter in the proximal tubule with elevated levels of FGF23 being closely associated with mortality.
Ref:https://link.springer.com/chapter/10.1007/978-3-319-10292-4_8
_______________________________________
B11. Haemodialysis (Charles)
Hemodialysis therapy has been one of the major breakthroughs in medicine in the twentieth century, allowing end-stage renal failure (ESRD) patients to remain alive for years or decades and to restore and continue their social and professional life and for some of them to wait for kidney transplantation. There has been since a tremendous effort in both clinical and engineering research to improve the burden of dialysis therapy and make it safer, easier, and more acceptable. In 50 years since Clyde Shields was started on chronic hemodialysis by Scribner et al. [1], a number of significant progresses have emerged such as the control of ultrafiltration, allowing to optimize convection, the way to assess the dialysis dose, the knowledge on uremic toxins, the release of more biocompatible and selectively permeable membranes, and the importance of nutrition for these patients. However despite all these significant improvements, we all face a persisting huge challenge because of the high mortality rate among dialysis patients, and questioning the current practices in the field of hemodialysis must be a continuous process: When to start dialysis therapy? How long and how frequent hemodialysis should be? Is high-volume convection the key? We owe the answers of these questions to our patients and their families and also to the healthcare authorities to provide the most cost-effective therapy.
Ref:https://link.springer.com/chapter/10.1007/978-3-319-10292-4_11
_______________________________________
B12- Peritoneal dialysis (Davies)
As is frequently pointed out, the record for clinical trials in patients treated with dialysis is poor, and this is especially the case for peritoneal dialysis. The reasons are complex and include a relatively small patient population, a lack of critical mass (with some exceptions) in trial methodology and infrastructure leading to an overreliance on industry support, the relatively complex outcomes and endpoints that affect patients treated with the modality, and a low repertoire of novel interventions. It would also be fair to say that the outcomes of patients treated with peritoneal dialysis over the last 30 years have improved considerably without these trials, largely because we have learned a lot about the therapy from a number of key observational studies and registry data analyses.
Ref:https://link.springer.com/chapter/10.1007/978-3-319-10292-4_12
_______________________________________
B13. Renal transplantation (Matter)
In kidney transplantation, the major step forward has been, in the 1970s and 1980s, the use of calcineurin inhibitors (CNIs), which dramatically reduce the number of acute rejections within the first year post transplantation. However, beyond this first year, the half-life of the kidney does not increase significantly compared to that in the pre-CNI era.
Ref:https://link.springer.com/chapter/10.1007/978-3-319-10292-4_13
_______________________________________











